Land pollution causes and effects are becoming a major global concern as they directly influence ecosystems, human health, and natural resources. The impacts of land pollution can be seen in declining soil fertility, loss of biodiversity, and contamination of water sources. Moreover, the soil contamination consequences of land pollution extend to food insecurity, habitat destruction, and long-term environmental instability. These effects of soil pollution are worsened by rapid industrialization, urban waste generation, and unsustainable land-use practices. As a result, the outcomes of land contamination now threaten the survival of humans and animals alike, disrupting ecological balance and reducing land productivity. The results of land degradation also lead to toxic exposure, reduced agricultural output, and weakened ecosystem resilience. Understanding these environmental effects of land pollution is essential for adopting better waste management, pollution control, and conservation measures to protect our land for future generations.
n the previous blog, we had seen an overview of the effects and causes of land pollution. In this blog, I will show you the effects of land pollution in detail and how it drastically affects the entire ecosystem and threatens the survival of our planet.
Causes and effects of land pollution
Land pollution is the degradation of the Earth’s land surface due to the accumulation of toxic substances harmful to both man and the ecosystem. The effects of land pollution don’t limit to soil pollution but have serious biological, ergonomic and economic consequences. Let’s have a look at the disastrous effects of land pollution.

Soil contamination consequence
Soil contamination consequence is a major environmental concern that affects the health and productivity of land. It occurs when harmful chemicals or pollutants alter the natural nutrient balance of topsoil. The soil contamination consequence becomes severe when excessive chemical fertilisers, uncontrolled soil erosion, and aggressive pest control methods degrade soil quality. Over time, the soil contamination consequence leads to reduced agricultural productivity, loss of forest cover, and declining pasturelands. This degradation ultimately threatens food security, biodiversity, and long-term land sustainability.
According to the World Wildlife Fund, half of the world’s topsoil has been destroyed in the previous 150 years. For 2.5 millimetres of topsoil, the regeneration process takes at least 500 years. Now you can imagine the magnitude of the crisis that we are undergoing.

According to the United Nations’ Food and Agriculture Organization (FAO), excessive use of chemical fertilisers and pesticides destroys essential soil microbes. Destruction of these microbes results in diminishing biodiversity and has disastrous repercussions for soil health. Microorganisms are required for several processes that contribute to soil fertility, including:
- Nutrient cycling, through which microbes convert nutrients into forms that crops can utilise.
- Microorganisms degrade hazardous substances that are by-products of agrochemicals, thereby reducing soil contamination. If there aren’t any microbes in the soil, it can drastically reduce its fertility.
Also read: What are Water Pollutants? – Definition, Sources and Types
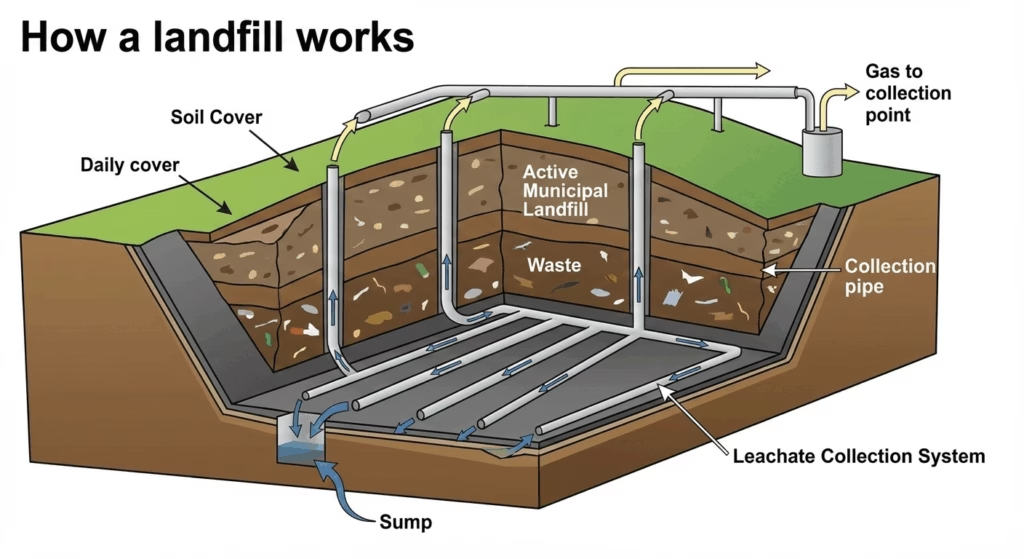
Poisoning of groundwater
When we inappropriately dispose of hazardous chemicals and other wastes on land or in illegal landfills, the chemicals may eventually seep into the groundwater system. This procedure is known as leaching. It can occur on farms, industrial sites, and landfills, posing a health risk to animals, plants, and humans.

Nutrient pollution, which arises due to farm runoff of chemical fertilisers is a subset of land pollution. The resulting nutrient enrichment of water bodies leads to eutrophication. When humans consume this contaminated water it leads to a variety of health issues. Even in little amounts, increased nitrate levels in water from fertiliser residues can be detrimental to newborns causing Blue Baby Syndrome.
The poisoning of groundwater which is a major source of drinking water for humans creates the worst consequence of land pollution, that is drinking water shortage. Let’s move on to it.
Drinking-Water shortage
Land pollution has the potential to spread in all directions, causing harm to the surrounding ecosystem. It can contaminate water and drastically impair its quality. Surface rainwater runoff carries chemicals and other harmful substances from landfills and solid wastes into rivers and makes the water unfit for human consumption.
Simultaneously, leaching occurs, allowing harmful metals and compounds to penetrate aquifers and water tables. Furthermore, contaminated water evaporates and condenses as precipitation along with the contaminants, perpetuating the pollution and aggravating the drinking water shortage.
Also read: Water Pollution – Effects and Causes
Land pollution and human health
Land pollution and human health are closely connected, as contaminated soil and waste exposure can lead to serious illnesses and long-term health risks. The effects of land pollution and human health include respiratory issues, skin diseases, neurological problems, and increased vulnerability to toxic chemicals in our environment.
Many parts of the world, particularly developing countries and slum areas, have massive amounts of rubbish piled up in the streets. This increases the contact of garbage with humans and the food we consume. These wastes contain hazardous chemicals, insecticides, and metals all of which are harmful to humans.

Plastic garbage contains chemicals like acrylic, polyvinyl chloride, polycarbonate, and phthalates. They have the potential to cause cancer, skin ailments, respiratory problems, and birth defects in pregnant people. Upon burning plastics in landfills, these chemicals escape into the air and pollute the air. In some other cases, they seep into water bodies. Ultimately, they reach the human body and damage the internal organs.
Chemical components contained in pharmaceuticals, pesticides and fertilisers, industrial wastes, such as cadmium, asbestos, mercury, cyanide, arsenic, and chromium, have severe impacts on human health. They are carcinogenic and can also cause lung, kidney, and liver damage.
According to a 2015 scientific study, “cancer villages” in China are connected to locations where farming takes place on land poisoned by the overuse of chemical pesticides and other heavy metals. Arsenic, asbestos, and dioxins are the main cause of cancer in Europe. Poisoning by lead and arsenic induce neurological damage and lower the IQ.
Habitat Loss
Wildlife creatures have suffered greatly in recent decades as a result of the continuous threats to their natural habitat and surroundings. Human commercial activities on land have gradually damaged and ruined the environment, forcing wildlife to migrate further away and adapt to new environments. As a result, some species have died while attempting to adapt, some have become extinct. And, others are on the verge of becoming extinct.

Air Pollution
Landfills and dump sites emit foul odours and stenches in the locations where they are located. Residents in cities and towns near large dump sites and landfill areas have reported excessive levels of a noxious odour. Aside from the unpleasant odour, landfills are constantly burning, contributing to air pollution.
Negative Impact on Tourism Industry
Landfills and abandoned waste disposal within cities generally create a negative picture of the population and the city’s governance. Landfills and garbage sites also degrade air quality and may pose a health risk to humans. As a result, it causes a city to lose its tourist appeal. This results in a loss of tourism revenue for the government.

Shall we wrap up?
Key Takeaways
- Land pollution has severe effects on ecosystems, animal, and human health due to toxic substances and industrial activities.
- Soil pollution reduces fertility and biodiversity, risking food production and disrupting essential microbial processes.
- Contaminated groundwater poses health risks, particularly through leaching of hazardous chemicals and agricultural runoff.
- Land pollution leads to water shortages, as harmful substances impair drinking water quality and availability.
- Ultimately, land pollution harms tourism and habitats, damaging local economies and threatening wildlife survival.
Conclusion
Land pollution effects create long-term damage that is often irreversible, especially when chemical residues cause soil contamination consequences that spread through land degradation from pollution. Once hazardous compounds enter the ground, the effects of land pollution on soil and groundwater intensify, posing serious land pollution and human health risks. Because the causes and effects of land pollution are interconnected, prevention remains the most effective solution. Increasing recycling, reducing misuse of land, and improving waste handling can limit further soil contamination consequences. Community cleanups and environmentally responsible practices also help control land degradation from pollution. By acting early and consistently, we can reduce the effects of land pollution on soil and protect both ecosystems and human health.