Eutrophication is a natural process in water bodies that becomes problematic when accelerated by human activities. It is caused by the excessive accumulation of nutrients, particularly nitrogen and phosphorus, leading to algal blooms and oxygen depletion. This phenomenon, also known as cultural eutrophication, occurs when agricultural runoff, wastewater discharge, and industrial effluents introduce pollutants into water systems. The cause of eutrophication is primarily linked to nutrient overloading, which fosters the growth of algae, eventually causing harm to aquatic ecosystems. The process of eutrophication disrupts aquatic life, while its effects include loss of biodiversity, poor water quality, and fish mortality. Understanding eutrophication causes and effects is crucial for managing its impact. In simple terms, eutrophication explained involves the imbalance caused by excess nutrients in aquatic systems.
Eutrophication in US freshwaters costs approximately $2.2 billion per year. Astonishing, right? Want to know more about this process that can wreak havoc if left unchecked? In this blog, let’s visit a eutrophied lake and understand the entire events that lead to eutrophication and its effects.
Let’s dive in.
- What Is Eutrophication?
- Eutrophication Process
- Types of Eutrophication
- Eutrophication Effects
- Eutrophication Control Measures
- Key Takeaways
- Conclusion
What Is Eutrophication?
Eutrophication refers to the process of nutrient over-enrichment in water bodies. It involves primarily nitrogen and phosphorus. This leads to excessive algal and plankton growth. This phenomenon, often termed cultural eutrophication, occurs due to human activities such as agricultural runoff and fertilizer use. Eutrophication explained highlights that this process reduces dissolved oxygen levels, causing poor water quality and threatening aquatic ecosystems.
The cause of eutrophication is primarily linked to artificial fertilizers and untreated waste discharge. As algae bloom, oxygen depletion follows, creating “dead zones” incapable of supporting life. This causes both environmental and ecological harm, ranking alongside global warming and deforestation. Understanding eutrophication meaning involves addressing the causes of eutrophication like synthetic fertilizers and urban runoff. The effects of eutrophication include biodiversity loss and water quality degradation, making it a critical issue in environmental management.
Also check out : Wastewater Treatment- Stages and Process full details.
In the next section, I will show you how a water body undergoes eutrophication.
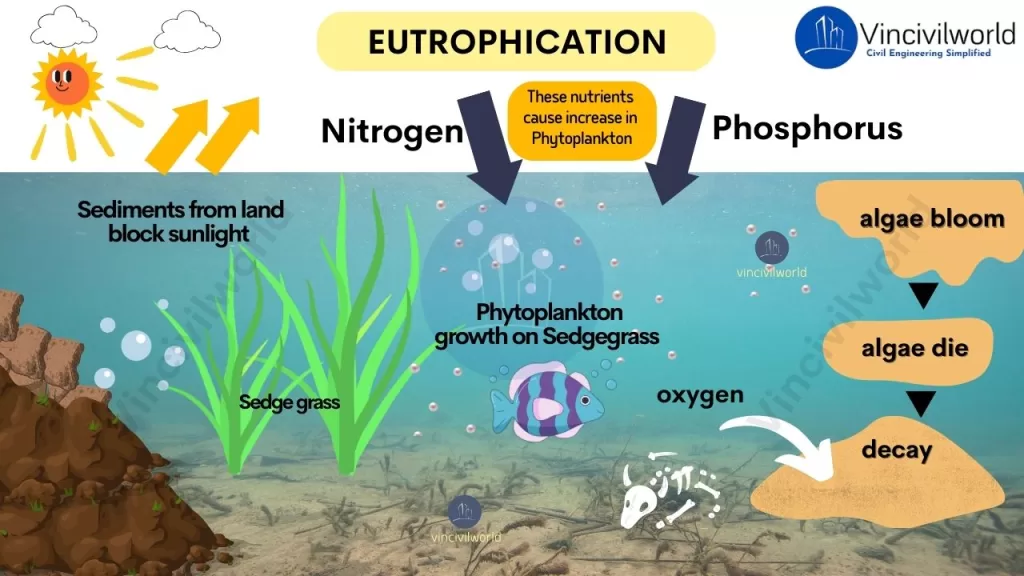
Eutrophication Process
- Soil receives nutrients in excess from synthetic fertilizers. Surface runoff washes them away into the water body.
- Nutrients reach the water body via untreated sewage and industrial effluents too.
- Excess nutrients cause accelerated growth of algae or algal bloom.
- Light penetration reduces due to the algal bloom.
- Plants beneath the algal bloom perish because they are unable to perform photosynthesis in the absence of sunshine.
- The algal bloom eventually dies and settles to the lake’s bottom.
- Bacterial populations begin to break down the remnants, consuming oxygen in the process.
- Oxygen is lost in the water as a result of decomposition.
- Aquatic organisms die due to a lack of dissolved oxygen. The waterbody turns into a dead zone which doesn’t support life.
Types of Eutrophication
Based on the source of nutrient enrichment, there are two types of eutrophication. They are:
Natural Eutrophication
Although human activities are the most prevalent cause of eutrophication, it can also be a natural process, especially in lakes. Due to climate change, geology and other external factors, the nutrient density of a water body increases over time and undergoes the process of natural eutrophication.
A few lakes also show the reverse process called meiotrophication. In this process, nutrient-poor inputs make the lake less nutrient-rich over time. Artificial lakes and reservoirs usually undergo this process, which starts out as very eutrophic but eventually become oligotrophic. An oligotrophic lake is a lake with low primary productivity due to low nutrient content.
The major difference between natural and anthropogenic eutrophication lies in the timescale. The former takes geologic ages to complete while the latter is a quick process.
Cultural Eutrophication
Eutrophication caused by human activity is also known as cultural or anthropogenic eutrophication. It is a process that accelerates natural eutrophication. Land runoff increases as a result of the land clearing and construction of towns and cities. Therefore, surface runoff from croplands carry nutrients such as phosphates and nitrate into the lakes and rivers, and then to coastal estuaries and bays.
When excess nutrients from anthropogenic sources such as runoff from fertilised croplands, lawns, and golf courses, untreated sewage and wastewater end up in water bodies, they cause nutrient pollution and simultaneously speeds up the natural process of eutrophication. The degradation of water quality induced by cultural eutrophication severely impacts human uses such as potable water, industrial usage, and recreation.
Let’s move on to the section describing its effects or consequences.
Eutrophication Effects
The effects of eutrophication range from ecological losses to economical losses. Let’s have a closer look at each one of them.
Loss of Biodiversity
Aquatic environments support a diverse range of plant and animal life. The process of eutrophication disrupts the ecosystem’s balance by promoting the growth of basic plant life. The ecosystem’s biodiversity is drastically reduced as a result of the loss of some desirable species.
The most noticeable consequence of cultural eutrophication is the formation of dense blooms of toxic, foul-smelling blue-green algae or cyanobacteria that impairs water clarity and quality. Algal blooms reduce light penetration. This limits aquatic plant growth and diminishes the success of predators that rely on light to hunt and catch prey in the benthic zone. Eventually, it leads to the mass death of aquatic plants and organisms.
Furthermore, eutrophication’s high rates of photosynthesis drain dissolved inorganic carbon and elevate pH to dangerously high levels throughout the day. By diminishing chemosensory skills, elevated pH can ‘blind’ organisms that use the sense of dissolved chemical cues for survival.
When the dense algal blooms die, microbial breakdown depletes dissolved oxygen, resulting in a hypoxic or anoxic “dead zone” where most species are unable to survive. Many freshwater lakes contain dead zones. Eutrophication-induced hypoxia (extremely low oxygen concentrations in bottom waters) and anoxia pose a danger to profitable commercial and recreational fisheries around the world.
Harmful Algal Blooms (HABs)
Some algal blooms are also dangerous because they produce toxins like microcystin and anatoxin-a. Harmful algal blooms (HABs) leads to:
- water quality degradation
- the extinction of commercially important fishes
- public health problems
Toxic cyanobacteria such as Anabaena, Cylindrospermopsis, Microcystis, and Oscillatoria (Planktothrix) dominate nutrient-rich, freshwater systems due to their superior competitive abilities under high nutrient concentrations, low nitrogen-to-phosphorus ratios, low light levels, reduced mixing, and high temperatures.
Toxic cyanobacteria bloom causes poisonings of domestic animals, wildlife, and even humans all around the world. For instance, shellfish poisoning is a result of HABs. Shellfish ingests the biotoxins produced during algal blooms. When humans consume them, it leads to various kinds of poisoning including paralytic, neurotoxic, and diarrhoetic shellfish poisoning.
Ciguatera, a predator fish becomes a vector for such toxins by accumulating the poison in its body and then poisoning the humans who consume it. Furthermore, cyanobacteria are responsible for various off-flavour compounds (such as methyl isoborneol and geosmin) detected in municipal drinking water systems.
Monetary Loss
Due to the continuous feeding of the fish, aquaculture ponds often accumulate high concentrations of nutrients such as nitrogen and phosphorus. As a result, these ponds are subjected to cyanobacterial blooms and hypoxia regularly. aquaculture-reared fish, resulting in significant financial losses.
Eutrophication also lowers the recreational value of rivers, lakes, and beaches. This severely impacts the tourism sector. When eutrophic conditions interfere with the treatment of drinking water, health concerns and monetary losses arise.
OK, I know what you’re thinking. How to control eutrophication, right? Read on to find more.
Eutrophication Control Measures
- Prevent the flow of plant nutrients to water bodies. Reduce the overuse of synthetic fertilizers.
- Proper channelling of agricultural wastes and runoffs.
- Releasing only safe and treated effluents to water bodies.
- Seaweed cultivation absorbs nitrogen and phosphorous and removes excess nutrients.
- Promoting the growth of shellfish.
That’s it about eutrophication. Hope you found it useful.
Key Takeaways
Eutrophication is a nutrient enrichment process in water bodies, primarily caused by nitrogen and phosphorus. While natural eutrophication takes centuries, cultural eutrophication accelerates due to human activities like agricultural runoff and untreated wastewater. This leads to algal blooms, oxygen depletion, and the formation of hypoxic or “dead zones,” harming aquatic ecosystems. Key effects include biodiversity loss, harmful algal blooms (HABs), water quality degradation, and economic losses in fisheries and recreation. Addressing eutrophication requires reducing nutrient pollution from fertilizers and industrial effluents. Understanding this process and its impacts is crucial for effective environmental management.
Conclusion
Eutrophication, whether natural or cultural, poses significant ecological and economic challenges. Cultural eutrophication, driven by human activities, intensifies nutrient pollution, causing harmful algal blooms and oxygen-depleted waters. The resulting biodiversity loss and water quality degradation lead to habitat destruction and economic losses, such as reduced fisheries and recreational opportunities. Combating eutrophication demands action, including limiting nutrient runoff, improving wastewater treatment, and promoting sustainable agricultural practices. By addressing these causes, we can mitigate the adverse effects on aquatic ecosystems and preserve water resources. Understanding eutrophication is key to safeguarding our environment and ensuring long-term ecological balance.