Azeotropes or azeotropic mixtures have always been a topic of interest due to their unique properties and the inability to separate them completely using conventional distillation. A classic example of azeotropes occurs in winemaking wherein an Ethanol-water mixture forms an azeotropic mixture at 96% Ethanol by volume which prohibits its further purification by distillation. In this blog, let’s look at how this happens and how we can separate such azeotropic mixtures.
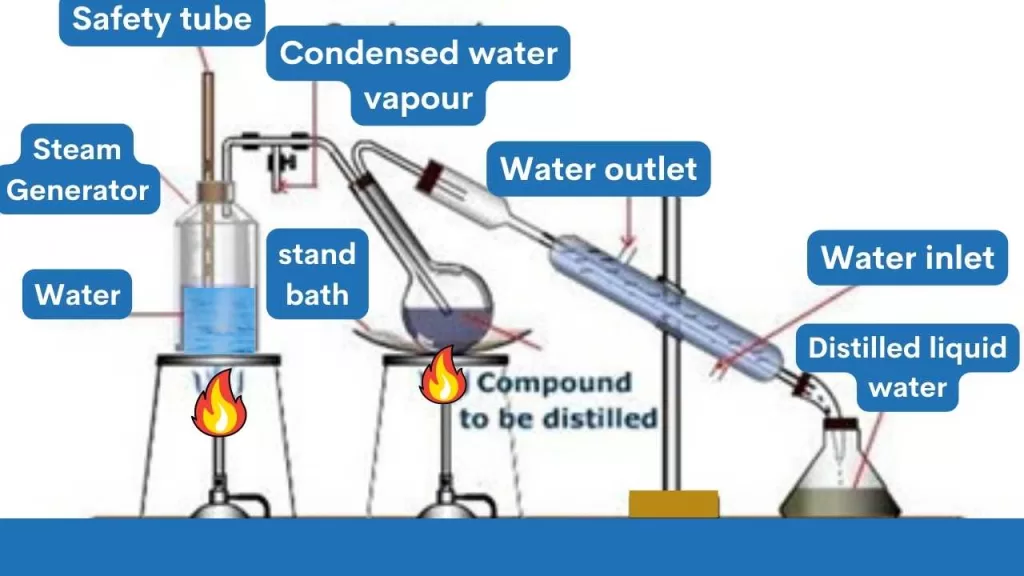
Before diving into azeotropes and azeotropic distillation, let’s have a quick look at the distillation process.
What are azeotropes or azeotropic mixtures?
Azeotropes are constant boiling point mixtures. Azeotropes are mixtures of two or more liquids whose composition cannot be altered or changed by simple distillation. This occurs because the vapour’s constituent ratios are identical to those of the unboiled mixture when an azeotrope is boiled. Azeotropes are also known as constant boiling point mixtures since distillation leaves their composition unaltered.
There is a distinctive boiling point for each azeotrope. An azeotrope’s boiling point is either lower or higher than the boiling points of any of its constituents. Depending on the boiling point deviation, we have two types of azeotropes as follows:
Maximum boiling azeotropes
Maximum boiling azeotropes are those mixtures that have a boiling point higher than any of their constituents. These azeotropes show a large negative deviation from Raoult’s Law. So we can call them negative azeotropes or pressure minimum azeotropes.
Hydrochloric acid at a concentration of 20.2% and 79.8% water (by mass) is an example of a negative azeotrope. Water and hydrogen chloride both boil at 100 °C and 84 °C, respectively, but the azeotrope boils at 110 °C, exceeding the boiling points of both of its ingredients. Any hydrochloric acid solution can boil at a maximum temperature of 110 °C. Other negative azeotropes include:
- Nitric acid (68%)/water, which boils at 120.2 °C at 1 atm
- Hydrofluoric acid (35.6%)/water, which boils at 111.35 °C
- Water with perchloric acid (71.6%), 203 °C boiling point
- Water and sulfuric acid (98.3%), boiling at 338 °C
More from Vincivilworld…
Minimum boiling azeotropes
Minimum boiling azeotropes are those mixtures that have a boiling point higher than any of their constituents. These azeotropes show a large positive deviation from Raoult’s Law. So we can call them positive azeotropes or pressure maximum azeotropes.
The mixture of 95.63% ethanol and 4.37% water (by mass), which boils at 78.2 °C, is a well-known example of a positive azeotrope. The azeotrope boils at 78.2 °C, which is lower than any of its components as ethanol boils at 78.4 °C and water boils at 100 °C.
Azeotropic Distillation
Since the vapours of azeotropes produced after boiling have the same composition as that of its liquid mixture, conventional distillation techniques can’t separate azeotropes. Hence we should add an additional component ie the entrainer, which can first break the existing azeotrope and make one of the components of the azeotrope more volatile than the other. In other words, azeotropic distillation is the process of converting a binary azeotrope into a ternary azeotrope by the addition of an entrainer.
An entrainer is a substance that we introduce to an azeotropic mixture to break it by changing the molecular interactions and creating a new azeotrope with a different composition and boiling point. The characteristics of the azeotropic mixture that undergoes separation determine the appropriate entrainer. The entrainer should be easily separable from the other components of the azeotropic mixture and form a new azeotrope with one of them.
The entrainer can change the activity coefficient of different compounds in different ways when added to the liquid phase, changing the relative volatility of a mixture. Greater deviations from Raoult’s law make it simpler to add another component and create considerable changes in relative volatility. In azeotropic distillation, the additional component has the same volatility as the mixture and one or more of the components combine to generate a new azeotrope due to polarity differences.
The most common types of entrainers in azeotropic distillation include:
- Extractive entrainers
- Azeotropic entrainers
Extractive Entrainers
Extractive entrainers are substances having a higher boiling point than the initial mixture and combining with one of the components in the azeotropic mixture to generate a new azeotrope. The mixture is heated after the addition of the extractive entrainer.
The extractive entrainer combines one of the original mixture’s components to create a new azeotrope as the mixture’s temperature rises. We can distill out the new azeotrope from the original mixture since it has a higher boiling point than the latter. A further distillation separates the entrainer from the isolated component.
Azeotropic Entrainers
Azeotropic entrainers are substances having a lower boiling point than the initial mixture and produces a new low boiling azeotrope. The most well-known example is the water – ethanol azeotrope when benzene or cyclohexane is added. The ternary azeotrope, which is 7% water, 17% ethanol, and 76% cyclohexane, boils at 62.1 °C with cyclohexane acting as the entrainer. The water/ethanol azeotrope is given just enough cyclohexane to engage all of the water in the ternary azeotrope. The azeotrope ( Benzene – water ) vaporises when the combination is then heated, leaving a residue that is almost entirely made up of ethanol.
Molecular Sieves
A common approach involves the use of molecular sieves. Treatment of 96% ethanol with molecular sieves gives anhydrous alcohol, the sieves having adsorbed water from the mixture. The sieves can be subsequently regenerated by dehydration using a vacuum oven.
Shall we wrap up?
Conclusion
In this blog, we had a short discussion on azeotropes, their formation, properties and the methods of separating them. Azeotropic distillation, pressure swing distillation and molecular sieves are some of the existing methods available. In case of any doubts, please feel free to ask in the comments section. Happy Learning!